Abstract
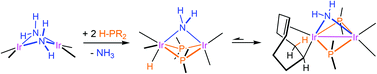
Selected secondary phosphanes (H–PR2; R = Ph, Cy, iPr) smoothly react with a parent amido-bridged diiridium cyclooctadiene complex affording mixed amido/(bis)phosphido dinuclear species. A careful investigation of the reaction profile, carried out by experimental and theoretical tools, revealed that, after an initial amido/phosphido exchange, at low temperatures a second molecule of secondary phosphane adds to the dinuclear system through an oxidative addition process leading to a hydrido amido/bis(phosphido) mixed-valence complex [IrIII/IrI]. These species rearrange above −10 °C into the most stable isomer that arises from a migration of the hydrido moiety to one of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH fragments of a coordinated cod molecule, a transformation facilitated by the formation of an intermetallic bond. Further heating of these species reductively eliminates ammonia affording bis(phosphido)-metal–metal bonded complexes.
CH fragments of a coordinated cod molecule, a transformation facilitated by the formation of an intermetallic bond. Further heating of these species reductively eliminates ammonia affording bis(phosphido)-metal–metal bonded complexes.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners

 Please wait while we load your content...
Please wait while we load your content...