Induced chirality-at-metal and diastereoselectivity at Δ/Λ-configured distorted square-planar copper complexes by enantiopure Schiff base ligands: combined circular dichroism, DFT and X-ray structural studies†
Abstract
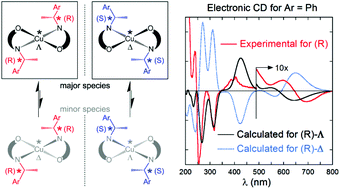
Bidentate enantiopure Schiff base ligands, (R or S)-N-1-(Ar)ethyl-2-oxo-1-naphthaldiminato-κ2N,O, diastereoselectively yield Δ/Λ-chiral four-coordinated, non-planar Cu(N^O)2 complexes [Ar = C6H5R/S-L1, m-C6H4OMe R-L2, p-C6H4OMe R/S-L3, and p-C6H4Br R/S-L4]. Two N,O-chelate ligands coordinate to the copper(II) atom in distorted square-planar mode, and induce metal-centered Δ/Λ-chirality at the copper atom in the C2-symmetric complexes. In the solid state, the R-L1 (or R-L4) ligand chirality diastereoselectively induces a Λ-Cu configuration in Λ-Cu-R-L1 (or Λ-Cu-R-L4), the S-L1 ligand a Δ-Cu configuration in Δ-Cu-S-L1, forming enantiopure crystals upon crystallization. Conversely, the R-L2 ligand combines both Λ/Δ-Cu-R-L2 as a diastereomeric pair in the crystals. In solution, electronic circular dichroism (CD) spectra show full or partial diastereoselectivity towards Λ-Cu for R ligands and towards Δ-Cu for S ligands. The electronic CD spectra measured on all complexes obtained from R ligands (or S ligands), e.g.Cu-R-L1, Cu-R-L2, Cu-R-L3, and Cu-R-L4 (or Cu-S-L1, Cu-S-L3, and Cu-S-L4), show consistent spectral features. TDDFT calculations of the electronic CD spectra for the diastereomers Λ-Cu-R-L1 and Δ-Cu-R-L1 suggest that the CD spectra are largely dominated by the configuration at the metal center (Λvs.Δ). The experimental CD spectrum of Cu-R-L1 agrees well with the one calculated for the Λ-Cu-R-L1 configuration. Cyclic voltammetry of Cu-R-L1 reveals a quasi-reversible redox wave corresponding to one-electron transfer for the [CuIIL2]0/[CuIL2]−1 couple in acetonitrile. DSC analyses for the complexes show an exothermic peak between 377 and 478 K (ΔH = −12 to −43 kJ mol−1), corresponding to a phase transformation from distorted square-planar/tetrahedral to regular tetrahedral geometry on heating.

 Please wait while we load your content...
Please wait while we load your content...