Unsymmetrical N-heterocyclic carbenes with a 1,1′-ferrocenediyl backbone†
Abstract
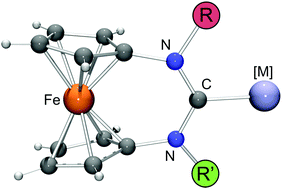
This paper focuses on ferrocene-based expanded-ring N-heterocyclic carbenes (NHCs) of the type [Fe(C5H4–NR–C–NR′–C5H4)] (1-R/R′), which contain two different N-substituents. Three combinations were addressed, with R = neopentyl (Np) in each case and R′ being either 2-adamantyl (Ad), phenyl (Ph) or 9-anthracenylmethyl (Acm). The NHCs were generated by reaction of the corresponding formamidinium tetrafluoroborates [H–1-R/R′][BF4] with lithium diisopropylamide (LDA). While only 1-Np/Ad was sufficiently stable for isolation, 1-Np/Ph and 1-Np/Acm could be efficiently trapped in situ by complexation reactions. Two series of RhI complexes were prepared, viz. [RhCl(cod)(1-R/R′)] (cod = 1,5-cyclooctadiene) by reacting [{Rh(μ-Cl)(cod)}2] with 1-R/R′ and cis-[RhCl(CO)2(1-R/R′)] by reacting [RhCl(cod)(1-R/R′)] with CO. All complexes exhibit pronounced anagostic α-CH⋯Rh interactions, both in solution and in the solid state, in accord with a strong influence of the N-substituents on the steric ligand properties, as is chemically illustrated by the huge reactivity difference of [RhCl(cod)(1-Ad)] (R = R′ = Ad) and [RhCl(cod)(1-Np/Ad)] towards CO, the former complex being inert. Tolman electronic parameter (TEP) values are 2050 ± 1 cm−1 for the unsymmetrical NHCs studied, indicating only a weak influence of the N-substituents on the electronic ligand properties.

 Please wait while we load your content...
Please wait while we load your content...