Easily available nickel complexes as catalysts for the intermolecular hydroamination of alkenes and alkynes†
Abstract
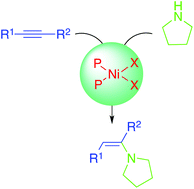
A series of nickel complexes of the type [(P–P)NiX2] ((P–P) = bisphospines or bisphosphites, X = chloride, triflate) were used as catalysts for the hydroamination of both activated and unactivated alkenes and alkynes with pyrrolidine. In general, the use of activated unsaturations, such as acrylonitrile, required mild reaction conditions (e.g. 100 °C and 4 h) in comparison with other non-activated alkenes. Particularly with a series of alkynes, the use of nickel(II) centers diminished or even inhibited the formation of otherwise undesired homocoupling and/or transfer hydrogenation by-products, such as the ones obtained in the presence of zerovalent nickel. When using less activated substrates, better selectivity was obtained, although harsher reaction conditions were needed. From a general perspective, the results of this report strongly support the potential use of nickel as a good candidate for further application in the hydroamination of organic unsaturations by means of screening of several π acceptor ligands.

 Please wait while we load your content...
Please wait while we load your content...