A family of cationic oxime-based hexametallic manganese(iii) single-molecule magnets†
Abstract
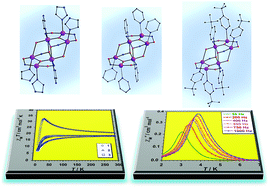
Three novel cationic hexametallic Mn(III) compounds of formulae [Mn6(μ3-O)2(H2N-sao)6(py)6(EtOH)2](ClO4)2·4EtOH (1), [Mn6(μ3-O)2(H2N-sao)6(tpy)6(H2O)2](ClO4)2·2tpy·4H2O·2EtOH (2) and [Mn6(μ3-O)2(H2N-sao)6(Him)6(EtOH)2](ClO4)2·6EtOH (3) [H2N-saoH2 = salicylamidoxime, py = pyridine, tpy = 4-tert-butylpyridine and Him = imidazole] have been synthesised and magnetostructurally characterised. These [Mn6]2+ complexes are new members of the oxime-based family of neutral [Mn6] single-molecule magnets (SMMs) in which the previously terminally bonded carboxylates, phosphinates, halides or ROH molecules on the outwardly facing triangular faces have been replaced with pyridine (1), 4-tert-butylpyridine (2) and imidazole (3) molecules generating a new series of salts based on Mn6 complexes. These results suggest that the cationic [Mn6]2+ species could be used as suitable building blocks for preparing new materials with different functionalities.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...