In search of redox non-innocence between a tetrazine pincer ligand and monovalent copper†
Abstract
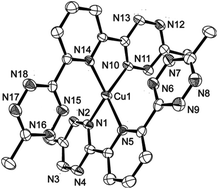
The synthesis and characterization of a “super-saturated” six-coordinate complex of monovalent copper (PF6− salt) and the potentially oxidizing pincer ligand 2,6, bis-(methyl tetrazinyl)pyridine, btzp, are described. This cation has a structure with the two pincers symmetry related, but each btzp having one short and one long Cu–N(tetrazine) bond; facile exchange is observed between short and long tetrazine donors. The structure shows no evidence of full electron transfer from Cu+ to tetrazine, and DFT calculations not only confirm this conclusion, but also show that the lowest energy triplet state, an excited state relative to the singlet ground state, has the MLCT character where the electron lost by copper now resides in both tetrazine rings of only one pincer ligand; the two pincers in this excited state are inequivalent, having charged btzp0 and btzp−1. The unusual orientation of the distinct tetrazines in the ground state structure of Cu(btzp)2+ is attributed to charge/dipole attraction unhindered by significant entropy cost.

 Please wait while we load your content...
Please wait while we load your content...