Dual role of 2-tosylaminomethylaniline as a ligand and a nucleophile in the copper-mediated oxidation of methanol†
Abstract
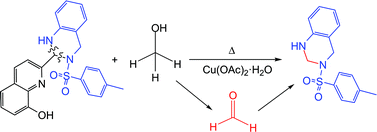
Some reactivity in the oxidation of methanol to formaldehyde has been spectroscopically detected on the methanolic mother liquors of a copper(II) complex of a Schiff base ligand derived from the condensation of 8-hydroxyquinoline-2-carboxaldehyde and 2-tosylaminomethylaniline. An investigation has shown that 2-tosylaminomethylaniline (HATS) plays a dual role in the oxidative process acting as a N-donor ligand and reacting in situ with formaldehyde, which leads to 3-tosyl-1,2,3,4-tetrahydroquinazoline (1). This was characterized by using both spectroscopic and X-ray diffraction techniques. The influence of 8-hydroxyquinoline derivatives, water and ligand stoichiometry on the yield of 1 was studied.

 Please wait while we load your content...
Please wait while we load your content...