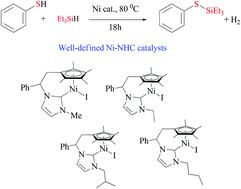
Dehydrogenative coupling of aromatic thiols with Et3SiH catalysed by N-heterocyclic carbene nickel complexes†
Abstract
A series of new tetramethylcyclopentadienyl-functionalised N-heterocyclic carbene ligands with different wingtip substituents have been prepared and characterised. These ligands have been successfully coordinated to nickel affording complexes of the general type (Cp*-NHCR)NiX (X = Cl, I). These well-defined nickel complexes selectively catalysed the coupling of aromatic thiols with Et3SiH to give the corresponding silylthioethers (RSSiEt3). The nickel complexes bearing ethyl, iso-butyl, and n-butyl wingtips displayed comparable catalytic efficiency, while the nickel complex bearing a methyl substituent on the wingtip was the worst performing catalyst.

 Please wait while we load your content...
Please wait while we load your content...