A DFT study of the mechanism of copper-catalyzed synthesis of 2H-indazoles from aryl azide†
Abstract
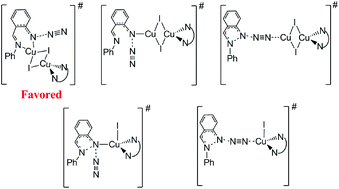
DFT calculations have been performed to study the reaction mechanism of N–N bond formation from aryl azide catalyzed by the copper(I) iodide complex. We studied various activation modes for the azide group, and found that the azide group is activated by the Cu(μ-I)2Cu(TMEDA) dimer coordinating to the N-atom of phenyl imine and the internal N-atom of azide.

 Please wait while we load your content...
Please wait while we load your content...