Synthesis and structure of N,C-chelated organoantimony(v) and organobismuth(v) compounds†
Abstract
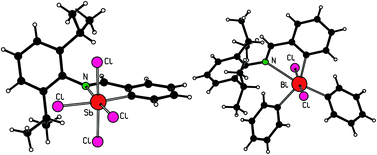
The reaction of N,C-intramolecularly coordinated organoantimony(III) and organobismuth(III) compounds LMCl2 (M = Sb (1) or Bi (2) and L = [o-(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-2,6-iPr2C6H3)C6H4]) with phenyllithium in a 1 : 1 or 1 : 2 molar ratio gave compounds LM(Ph)Cl (M = Sb (3) or Bi (4)) and LMPh2 (M = Sb (5) or Bi (6)) in moderate to good yields. Compound 3 could also be prepared by the treatment of the lithium compound LLi with in situ prepared PhSbCl2. Oxidation of the antimony(III) compounds 1, 3 and 5 with one equivalent of SO2Cl2 proceeded smoothly with formation of organoantimony(V) compounds LSbCl4 (7), LSb(Ph)Cl3 (8) and LSbPh2Cl2 (9) in nearly quantitative yields. Compounds 7–9 are yellowish solids that are stable for a long time even in the presence of air. In contrast, only organobismuth(III) compounds 4 and 6 could be successfully oxidized using SO2Cl2 to give compounds LBi(Ph)Cl3 (10) and LBiPh2Cl2 (11). Compound 11 is stable, but compound 10 readily decomposed in solution and could not be isolated and stored for a longer period. All attempts to prepare compound LBiCl4 by the oxidation of 2 with SO2Cl2 failed and resulted only in a mixture of products. All studied compounds were characterized by electrospray ionization (ESI) mass spectrometry, and 1H and 13C NMR spectroscopy. The molecular structures of 3–7, 9 and 11 were unambiguously established using single-crystal X-ray diffraction analysis.
N-2,6-iPr2C6H3)C6H4]) with phenyllithium in a 1 : 1 or 1 : 2 molar ratio gave compounds LM(Ph)Cl (M = Sb (3) or Bi (4)) and LMPh2 (M = Sb (5) or Bi (6)) in moderate to good yields. Compound 3 could also be prepared by the treatment of the lithium compound LLi with in situ prepared PhSbCl2. Oxidation of the antimony(III) compounds 1, 3 and 5 with one equivalent of SO2Cl2 proceeded smoothly with formation of organoantimony(V) compounds LSbCl4 (7), LSb(Ph)Cl3 (8) and LSbPh2Cl2 (9) in nearly quantitative yields. Compounds 7–9 are yellowish solids that are stable for a long time even in the presence of air. In contrast, only organobismuth(III) compounds 4 and 6 could be successfully oxidized using SO2Cl2 to give compounds LBi(Ph)Cl3 (10) and LBiPh2Cl2 (11). Compound 11 is stable, but compound 10 readily decomposed in solution and could not be isolated and stored for a longer period. All attempts to prepare compound LBiCl4 by the oxidation of 2 with SO2Cl2 failed and resulted only in a mixture of products. All studied compounds were characterized by electrospray ionization (ESI) mass spectrometry, and 1H and 13C NMR spectroscopy. The molecular structures of 3–7, 9 and 11 were unambiguously established using single-crystal X-ray diffraction analysis.

 Please wait while we load your content...
Please wait while we load your content...