Improved O2 evolution from a water splitting reaction over Er3+ and Y3+ co-doped tetragonal BiVO4
Abstract
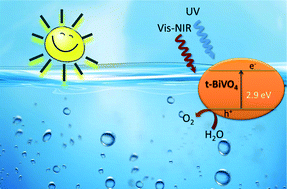
Erbium–yttrium co-doped BiVO4 with a tetragonal structure is synthesized by means of a surfactant free hydrothermal method. The studied photocatalyst shows good photoactivity under sun-like excitation for the degradation of methylene blue (MB) and for O2 evolution. From structural and morphological characterization, it has been stated that the presence of lanthanides induces the stabilization of the tetragonal phase. This is probably due to the substitutional occupation that occurs in the BiVO4 lattice. The photocatalytic performance under visible-NIR radiation clearly evidences the occurrence of an up-conversion process involved in the overall photo-electronic mechanism. The tetragonal phase Er0.0075,Y0.03–Bi0.9625VO4 system gives the highest O2 evolution rate (425 μmol g−1 h−1) under sun-like excitation, being 8 times higher than that attained for m-BiVO4 (53 μmol g−1 h−1).

 Please wait while we load your content...
Please wait while we load your content...