Tuning the hydrogenation activity of Pd NPs on Al–MIL-53 by linker modification†
Abstract
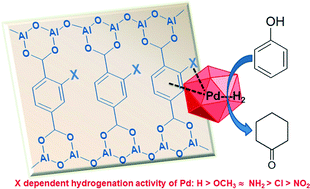
The hydrogenation activity of 3 wt.% Pd nanoparticles supported on various mono-group (H, OCH3, NH2, Cl, and NO2) substituted Al–MIL-53 materials has been investigated. Substituents enhanced the dispersion of palladium nanoparticles on Al–MIL-53, leading to a narrow particle size distribution in the range of 2 to 4 nm. Pd nanoparticles on fresh catalysts were present as a mixture of Pd(II) and Pd(0) with different ratios. These Pd species readily became metallic in a hydrogen flow even at room temperature. Their activities in hydrogenation of phenol and phenylacetylene are linked to the substituents on the aromatic ring of the framework. Catalysts with electron-donating groups (OCH3 and NH2) show much higher activity than those containing electron-withdrawing groups (Cl and NO2). This behavior might be explained by the hydrogen dissociation abilities of metallic Pd nanoparticles affected by the organic linkers.

 Please wait while we load your content...
Please wait while we load your content...