The synthesis of pyrroles via acceptorless dehydrogenative condensation of secondary alcohols and 1,2-amino alcohols mediated by a robust and reusable catalyst based on nanometer-sized iridium particles†
Abstract
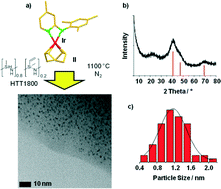
Pyrroles are important compounds with several applications in medicine and material science. They can be synthesized sustainably from secondary alcohols and amino alcohols. Hydrogen and water are liberated in the course of this reaction. Here, we present that this sustainable catalytic pyrrole synthesis can be mediated efficiently by a novel iridium nanoparticle catalyst. The catalyst synthesis starts from molecular precursors, an N-ligand stabilized Ir complex and a commercially available polysilazane. The generation of nanometer-sized iridium particles was achieved (due to the presence of N atoms in the support). The robust nature of the support allows reuse of the catalyst. The scope of the reaction was verified by the synthesis of 23 pyrrole derivatives (up to 93% isolated yield). Thus, an attractive functional group tolerance (e.g. amines and olefins) could be observed. Commercially available heterogeneous Ir catalysts are inefficient in this pyrrole synthesis and extremely limited in terms of reusability.

 Please wait while we load your content...
Please wait while we load your content...