CO oxidation on single and multilayer Pd oxides on Pd(111): mechanistic insights from RAIRS
Abstract
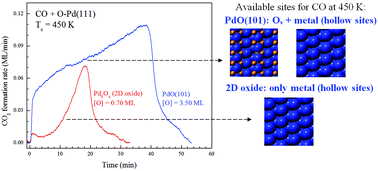
We investigated the oxidation of CO on the Pd5O4 surface oxide and a multilayer PdO(101) film on Pd(111) using direct, isothermal rate measurements, reflection absorption infrared spectroscopy (RAIRS) and density functional theory calculations. Our results show that the PdO(101) film is intrinsically more reactive than the surface oxide toward CO oxidation, and that the reaction kinetics occurs autocatalytically on both oxides, with the rate of CO2 production increasing as the oxides are reduced. The RAIRS measurements reveal that self-accelerating reaction kinetics occurs on PdO(101) because oxide reduction generates both oxygen vacancies within PdO(101) domains as well as Pd(111) domains. These reduced structures bind CO more strongly than the pristine PdO(101) surface, causing the CO surface concentration and thus the reaction rate to increase as the oxide is progressively reduced by reaction. In contrast, we find that reduction of the surface oxide generates only Pd(111) domains which bind CO strongly and sustain reaction.
- This article is part of the themed collection: Catalysis in the USA

 Please wait while we load your content...
Please wait while we load your content...