The effect of oxide acidity on HMF etherification†
Abstract
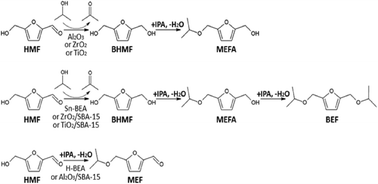
The liquid-phase (69 bar) reaction of 5-hydroxymethylfurfural (HMF) with 2-propanol for production of furanyl ethers was studied at 413 and 453 K over a series of oxide catalysts, including γ-Al2O3, ZrO2, TiO2, Al2O3/SBA-15, ZrO2/SBA-15, TiO2/SBA-15, H-BEA, and Sn-BEA. The acidity of each of the catalysts was first characterized for Brønsted sites using TPD-TGA of 2-propanamine and for Lewis sites using TPD-TGA of 1-propanol. Catalysts with strong Brønsted acidity (H-BEA and Al2O3/SBA-15) formed 5-[(1-methylethoxy)methyl]furfural with high selectivities, while materials with Lewis acidity (γ-Al2O3, ZrO2, TiO2, and Sn-BEA) or weak Brønsted acidity (ZrO2/SBA-15 and TiO2/SBA-15) were active for transfer hydrogenation from the alcohol to HMF to produce 2,5-bis(hydroxymethyl)furan, with subsequent reactions to the mono- or di-ethers. Each of the catalysts was stable under the flow-reactor conditions but the selectivities varied with the particular oxide being investigated.

 Please wait while we load your content...
Please wait while we load your content...