Catalytic selective oxidation of isobutane over Csx(NH4)3−xHPMo11VO40 mixed salts†
Abstract
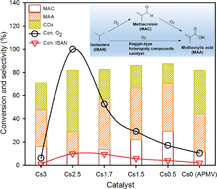
A series of mixed Keggin-type heteropolysalts Csx(NH4)3−xHPMo11VO40 with various ammonia/caesium ratios was prepared by the precipitation method and characterized by TGA, N2 adsorption/desorption, XRD, FT-IR, and NH3-TPD techniques. Correlations between the ammonia/caesium ratio and the specific surface area, as well as with the total number of acid sites, were established. Furthermore, the introduction of Cs to the catalytic formulation was beneficial to the stabilization of the Keggin structure and helped limiting the elimination of the V atoms from the primary structure. The as-prepared samples were applied in the catalytic selective oxidation of isobutane at 340 °C under atmospheric pressure. The best results were obtained over Cs1.7(NH4)1.3HPMo11VO40 with an isobutane conversion of 9.6% and a total selectivity to valuable products (methacrylic acid and methacrolein) of 57.1%. This was explained by the well-balanced acidity and specific surface of this catalyst, promoting the C–H bond activation (adequate acid properties) over a large number of accessible active sites (high acid sites density).

 Please wait while we load your content...
Please wait while we load your content...