Oxidation of hydrocarbons with H2O2/O2 catalyzed by osmium complexes containing p-cymene ligands in acetonitrile†
Abstract
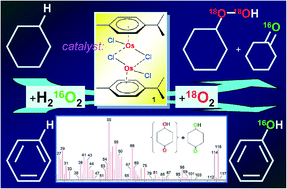
The soluble osmium complexes containing p-cymene (π-p-cym) ligands, [(η6-p-cym)OsCl2]2 (1), [(η6-p-cym)Os(bipy)Cl]PF6 (2), and [(η6-p-cym)2Os2(μ-H)3]PF6 (3), are efficient catalysts for the oxidation of alkanes (cyclohexane, n-heptane, methylcyclohexane, isooctane, and cis- and trans-1,2-dimethylcyclohexane) with hydrogen peroxide in air to the corresponding alkyl hydroperoxides in acetonitrile solution if a small amount of pyridine is present in the solution. The binuclear complex 1 is the most active precatalyst in the oxidation whereas compound 2 containing the bipyridine ligand is much less efficient. The oxidation of cyclohexane at 60 °C and low concentration [1]0 = 10−7 M gave a turnover number (TON) of 200 200 after 24 h. A study of the selectivity parameters in the oxidation of linear and branched alkanes and the kinetic peculiarities of the cyclohexane oxidation led to the conclusion that the main reaction mechanism includes the formation of hydroxyl radicals. The effective activation energy Ea for the cyclohexane oxidation catalyzed by complex 1 was 10 ± 2 kcal mol−1. A kinetic analysis verified also that monomerization of complex 1 occurs before the oxidizing species is involved in the catalytic cycle. The 1-catalyzed reaction of cyclohexane, c-C6H12, with H216O2 in an atmosphere of 18O2 gave labeled cyclohexyl hydroperoxide, c-C6H11–18O–18OH. In addition, a small amount of “light” cyclohexanone, c-C6H10![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 16O, is produced apparently via a mechanism which includes neither hydroxyl radicals nor incorporation of molecular oxygen from the atmosphere. The oxidation of benzene with H216O2 under 18O2 gave phenol which did not contain the 18O isotope. The reactions with cyclohexane and benzene were shown to proceed also via an alternative minor mechanism with oxo derivatives of high-valent osmium “Os
16O, is produced apparently via a mechanism which includes neither hydroxyl radicals nor incorporation of molecular oxygen from the atmosphere. The oxidation of benzene with H216O2 under 18O2 gave phenol which did not contain the 18O isotope. The reactions with cyclohexane and benzene were shown to proceed also via an alternative minor mechanism with oxo derivatives of high-valent osmium “Os![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O” as key oxidizing species.
O” as key oxidizing species.

 Please wait while we load your content...
Please wait while we load your content...