Influence of preparation method on supported Cu–Ni alloys and their catalytic properties in high pressure CO hydrogenation
Abstract
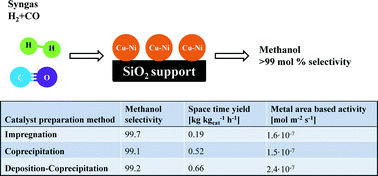
Silica supported Cu–Ni (20 wt% Cu + Ni on silica, molar ratio of Cu/Ni = 2) alloys are prepared via impregnation, coprecipitation, and deposition–coprecipitation methods. The approach to co-precipitate the SiO2 from Na2SiO3 together with metal precursors is found to be an efficient way to prepare high surface area silica supported catalysts (BET surface area up to 322 m2 g−1, and metal area calculated from X-ray diffraction particle size up to 29 m2 g−1). The formation of bimetallic Cu–Ni alloy nanoparticles has been studied during reduction using in situ X-ray diffraction. Compared to impregnation, the coprecipitation and deposition–coprecipitation methods are more efficient for preparation of small and homogeneous Cu–Ni alloy nanoparticles. In order to examine the stability of Cu–Ni alloys in high pressure synthesis gas conversion, they have been tested for high pressure CO hydrogenation (50 bar CO and 50 bar H2). These alloy catalysts are highly selective (more than 99 mol%) and active for methanol synthesis; however, loss of Ni caused by nickel carbonyl formation is found to be a serious issue. The Ni carbonyl formation should be considered, if Ni-containing catalysts (even in alloyed form) are used under conditions with high partial pressure of CO.

 Please wait while we load your content...
Please wait while we load your content...