Cation exchange at the secondary building units of metal–organic frameworks
Abstract
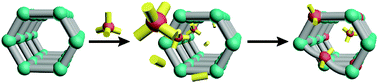
Cation exchange is an emerging synthetic route for modifying the secondary building units (SBUs) of metal–organic frameworks (MOFs). This technique has been used extensively to enhance the properties of nanocrystals and molecules, but the extent of its applications for MOFs is still expanding. To harness cation exchange as a rational tool, we need to elucidate its governing factors. Not nearly enough experimental observations exist for drawing these conclusions, so we provide a conceptual framework for approaching this task. We address which SBUs undergo exchange, why certain ions replace others, how the framework influences the process, the role of the solvent, and current applications. Using these guidelines, certain trends emerge from the available data and missing experiments become obvious. If future studies follow this framework, then a more comprehensive body of observations will furnish a deeper understanding of cation exchange and inspire future applications.
- This article is part of the themed collection: Metal Organic Frameworks (MOFs)

 Please wait while we load your content...
Please wait while we load your content...