The activation strain model and molecular orbital theory: understanding and designing chemical reactions
Abstract
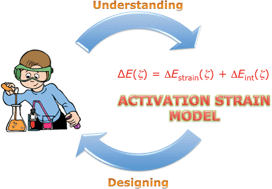
In this Tutorial Review, we make the point that a true understanding of trends in reactivity (as opposed to measuring or simply computing them) requires a causal reactivity model. To this end, we present and discuss the Activation Strain Model (ASM). The ASM establishes the desired causal relationship between reaction barriers, on one hand, and the properties of reactants and characteristics of reaction mechanisms, on the other hand. In the ASM, the potential energy surface ΔE(ζ) along the reaction coordinate ζ is decomposed into the strain ΔEstrain(ζ) of the reactants that become increasingly deformed as the reaction proceeds, plus the interaction ΔEint(ζ) between these deformed reactants, i.e., ΔE(ζ) = ΔEstrain(ζ) + ΔEint(ζ). The ASM can be used in conjunction with any quantum chemical program. An analysis of the method and its application to problems in organic and organometallic chemistry illustrate the power of the ASM as a unifying concept and a tool for rational design of reactants and catalysts.
- This article is part of the themed collection: Applied Computational Chemistry

 Please wait while we load your content...
Please wait while we load your content...