Redox catalysis in organic electrosynthesis: basic principles and recent developments
Abstract
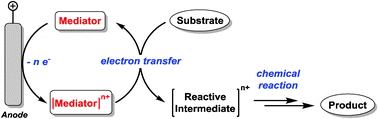
Electroorganic synthesis has become an established, useful, and environmentally benign alternative to classic organic synthesis for the oxidation or the reduction of organic compounds. In this context, the use of redox mediators to achieve indirect processes is attaining increased significance, since it offers many advantages compared to a direct electrolysis. Kinetic inhibitions that are associated with the electron transfer at the electrode/electrolyte interface, for example, can be eliminated and higher or totally different selectivity can be achieved. In many cases, a mediated electron transfer can occur against a potential gradient, meaning that lower potentials are needed, reducing the probability of undesired side-reactions. In addition, the use of electron transfer mediators can help to avoid electrode passivation resulting from polymer film formation on the electrode surface. Although the principle of indirect electrolysis was established many years ago, new, exciting and useful developments continue to be made. In recent years, several new types of redox mediators have been designed and examined, a process that can be accomplished more efficiently and purposefully using modern computational tools. New protocols including, the development of double mediatory systems in biphasic media, enantioselective mediation and heterogeneous electrocatalysis using immobilized mediators have been established. Furthermore, the understanding of mediated electron transfer reaction mechanisms has advanced. This review describes progress in the field of electroorganic synthesis and summarizes recent advances.

 Please wait while we load your content...
Please wait while we load your content...