Electrochemical in battery polymerization of poly(alkylenedioxythiophene) over lithium iron phosphate for high-performance cathodes†
Abstract
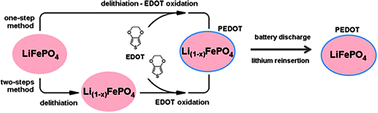
A molecular wiring concept was induced in LiFePO4 cathodes by in battery polymerization methods. This was performed by the addition of alkylthiophene monomers over the LiFePO4-based cathode during the first charging step in lithium test cells. The driving force for the in battery polymerization of the monomers was supplied by the oxidizing current and by the physical contact of monomers with delithiated Li1−xFePO4 formed during the charging of the battery. The resulting molecularly engineered cathodes give higher initial capacity, superior rate capability and improved cyclability compared to the pristine LiFePO4 compound. Further to observe changes in the oxidation state of iron, Mössbauer spectroscopy was employed and the results were correlated with those of impedance spectroscopy, which reveal a significant increase in conductivity during charging. The presented methods provide simple yet effective routes for manufacturing efficient cathode materials at room temperature, without the need of additional oxidizing compounds to carry out the polymerization process and to rival high temperature based carbon coatings.

 Please wait while we load your content...
Please wait while we load your content...