Benzene⋯acetylene: a structural investigation of the prototypical CH⋯π interaction†
Abstract
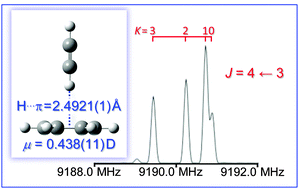
The structure of a prototype CH⋯π system, benzene⋯acetylene, has been determined in the gas phase using Fourier-transform microwave spectroscopy. The spectrum is consistent with an effective C6v structure with an H⋯π distance of 2.4921(1) Å. The HCCH subunit likely tilts by ∼5° from the benzene symmetry axis. The dipole moment was determined to be 0.438(11) D from Stark effect measurements. The observed intermolecular distance is longer than in similar benzene⋯HX complexes and than the distances observed in the benzene⋯HCCH cocrystal and predicted by many high level ab initio calculations; however, the experimentally estimated binding energy of 7.1(7) kJ mol−1 is similar to previously studied benzene⋯HX complexes. Several additional sets of transitions were observed in the rotational spectrum, likely corresponding to excited states arising from low energy intermolecular vibrational modes of the dimer.

 Please wait while we load your content...
Please wait while we load your content...