Combining experiment and theory to elucidate the role of supercritical water in sulfide decomposition†
Abstract
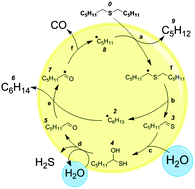
The cleavage of C–S linkages plays a key role in fuel processing and organic geochemistry. Water is known to affect these processes, and several hypotheses have been proposed, but the mechanism has been elusive. Here we use both experiment and theory to demonstrate that supercritical water reacts with intermediates formed during alkyl sulfide decomposition. During hexyl sulfide decomposition in supercritical water, pentane and CO + CO2 were detected in addition to the expected six carbon products. A multi-step reaction sequence for hexyl sulfide reacting with supercritical water is proposed which explains the surprising products, and quantum chemical calculations provide quantitative rates that support the proposed mechanism. The key sequence is cleavage of one C–S bond to form a thioaldehyde via radical reactions, followed by a pericyclic addition of water to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond to form a geminal mercaptoalcohol. The mercaptoalcohol decomposes into an aldehyde and H2S either directly or via a water-catalyzed 6-membered ring transition state. The aldehyde quickly decomposes into CO plus pentane by radical reactions. The time is ripe for quantitative modelling of organosulfur reaction kinetics based on modern quantum chemistry.
S bond to form a geminal mercaptoalcohol. The mercaptoalcohol decomposes into an aldehyde and H2S either directly or via a water-catalyzed 6-membered ring transition state. The aldehyde quickly decomposes into CO plus pentane by radical reactions. The time is ripe for quantitative modelling of organosulfur reaction kinetics based on modern quantum chemistry.

 Please wait while we load your content...
Please wait while we load your content...