Photocatalytic reduction of CO2 into hydrocarbon solar fuels over g-C3N4–Pt nanocomposite photocatalysts
Abstract
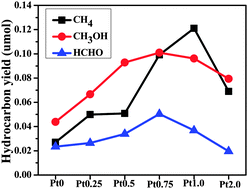
Photocatalytic reduction of CO2 into renewable hydrocarbon fuels is an alternative way to develop reproducible energy, which is also a promising way to solve the problem of the greenhouse effect. In this work, graphitic carbon nitride (g-C3N4) was synthesized by directly heating thiourea at 550 °C and then a certain amount of Pt was deposited on it to form g-C3N4–Pt nanocomposites used as catalysts for photocatalytic reduction of CO2 under simulated solar irradiation. The main products of photocatalysis were CH4, CH3OH and HCHO. The deposited Pt acted as an effective cocatalyst, which not only influenced the selectivity of the product generation, but also affected the activity of the reaction. The yield of CH4 first increased upon increasing the amount of Pt deposited on the g-C3N4 from 0 to 1 wt%, then decreased at 2 wt% Pt loading. The production rates of CH3OH and HCHO also increased with the content of Pt increasing from 0 to 0.75 wt% and the maximum yield was observed at 0.75 wt%. The Pt nanoparticles (NPs) could facilitate the transfer and enrichment of photogenerated electrons from g-C3N4 to its surface for photocatalytic reduction of CO2. At the same time, Pt was also used a catalyst to promote the oxidation of products. The transient photocurrent response further confirmed the proposed photocatalytic reduction mechanism of CO2. This work indicates that the deposition of Pt is a good strategy to improve the photoactivity and selectivity of g-C3N4 for CO2 reduction.

 Please wait while we load your content...
Please wait while we load your content...