Faster photoinduced electron transfer in a diluted mixture than in a neat donor solvent: effect of excited-state H-bonding†
Abstract
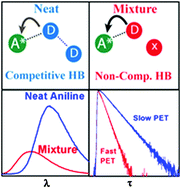
In a neat electron-donating solvent (in this case aniline), photoinduced electron transfer (PET) from the solvent to an excited acceptor (e.g. a coumarin fluorophore) may be anticipated to be the most efficient because of the close contact of the acceptor with many donors. Addition of an inert component would most likely retard the PET process by replacing some donors from the neighbourhood of the acceptors. Surprisingly, we found dramatic acceleration of PET (6–10 fold enhancement compared to neat aniline), for coumarin 102 (C102) dissolved in a binary mixture of aniline and an inert solvent (cyclohexane or toluene). The PET induced fluorescence follows an anomalous trend against the mole fraction of aniline (XAN); first quenches up to certain XAN (0.075 for cyclohexane; 0.13 for toluene), thereafter, enhances with increase in XAN. Although the non-interacting component cannot directly participate in the PET process, it may modulate C102–aniline H-bonding association by changing the polarity of the medium or by disrupting the aniline–aniline H-bond. The study clearly illustrates the dominant role of hydrogen bonding in activating the electron transfer rate where standard thermodynamics predicts very weak donor–acceptor interaction.

 Please wait while we load your content...
Please wait while we load your content...