Shear dynamics of nanoconfined ionic liquids
Abstract
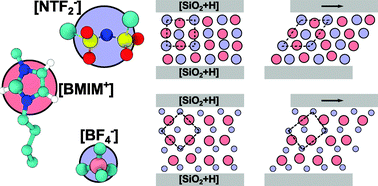
We used molecular dynamics simulations to study the structure and shear dynamics of two ionic liquids (ILs) featuring the same cation 1-butyl-3-methyl-imidazolium or [BMIM], paired with bis(trifluoromethanesulphonyl)amide [NTF2] and tetrafluoroborate [BF4] anions, confined between two hydroxylated silica surfaces. The results demonstrate how the shape of IL molecules affects their layering structure at hydroxylated silica surfaces and how the layered structure of nanoconfined liquids determines their dynamical properties at the molecular level. When [BMIM][NTF2] is sheared, larger molecular fluctuations in the inner layers are required to stabilise the system, and the resulting dynamics is irregular. The alternating charged layers in [BMIM][BF4] allow the system to stabilise through smaller oscillations, and the layers appear to shear on top of each other in a laminar fashion. The simulated dynamics explains qualitatively the relative change in viscosity that the two ILs exhibit when confined, as has been observed in previous experiments.

 Please wait while we load your content...
Please wait while we load your content...