A unified model for surface electrocatalysis based on observations with enzymes
Abstract
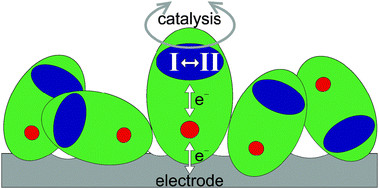
Despite being so large, many enzymes are not only excellent electrocatalysts – making possible chemical transformations under almost reversible conditions – but they also facilitate our understanding of electrocatalysis by allowing complex processes to be dissected systematically. The electrocatalytic voltammograms obtained for enzymes attached to an electrode expose fundamental aspects of electrocatalysis that can be addressed in ways that are not available to conventional molecular or surface electrocatalysts. The roles of individual components, each characterisable by diffraction or spectroscopy, can be tested and optimised by genetic engineering. Importantly, unlike small-molecule electrocatalysts (RMM < 1000) that are structurally well-defined but invariably altered by being attached to a surface, the enzyme is a giant, multi-component assembly in which the active site is buried and relatively insensitive to the presence of the electrode and solvent interface. A central assertion is that for a given driving force (electrode potential) a true catalyst has no influence on the direction of the reaction; consequently, ‘catalytic bias’, i.e. the common observation that an enzyme or indeed any electrocatalyst operates preferentially in one direction, must arise from secondary effects beyond the elementary catalytic cycle. This Perspective highlights and extends a general model for electrocatalysis by surface-confined enzymes, and explains how two secondary effects control the bias: (i) the electrode potential at which electrons enter or leave the catalytic cycle; (ii) potential-dependent interconversions between states of the catalyst differing in catalytic activity due to changes in the composition and arrangements of atoms. The model, which is easily applied to enzymes that have been studied recently, highlights important considerations for understanding and developing surface-confined electrocatalysts.
- This article is part of the themed collection: Photosynthesis: From Natural to Artificial

 Please wait while we load your content...
Please wait while we load your content...