Hydrogen defects in tetragonal ZrO2 studied using density functional theory†
Abstract
In the energy-structure paradigm, we analyzed the defects that can arise in tetragonal zirconium oxide (T-ZrO2) involving the hydrogen atom or the hydrogen molecule using density functional theory. Our results indicate that the dominant hydrogen defect under reducing conditions is 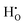 , a complex formed between the hydride ion and a doubly charged oxygen vacancy. This result is consistent with the experimental observation that under reducing conditions, the solubility of hydrogen is proportional to the degree of hypostoichiometry of T-ZrO2. Under oxidizing conditions we found three different hydrogen defects, each predominating in a specific range of the chemical potential of electrons. Starting from the valence band top toward the conduction band bottom, these defects are the interstitial proton,
, a complex formed between the hydride ion and a doubly charged oxygen vacancy. This result is consistent with the experimental observation that under reducing conditions, the solubility of hydrogen is proportional to the degree of hypostoichiometry of T-ZrO2. Under oxidizing conditions we found three different hydrogen defects, each predominating in a specific range of the chemical potential of electrons. Starting from the valence band top toward the conduction band bottom, these defects are the interstitial proton, 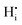 , a complex formed between two hydrogen species and a zirconium vacancy with a net effective charge of (2−),
, a complex formed between two hydrogen species and a zirconium vacancy with a net effective charge of (2−), 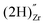 , and finally a complex similar to the latter but with a net effective charge of (4−),
, and finally a complex similar to the latter but with a net effective charge of (4−), 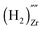 . In
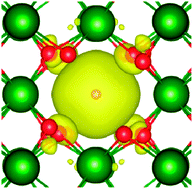
. In 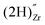 the two hydrogens exist in the form of hydroxyl groups, while in
the two hydrogens exist in the form of hydroxyl groups, while in 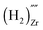 they exist in the form of a hydrogen molecule. In addition, we found that up to three hydrogen species can favorably accumulate in a zirconium vacancy under oxidizing conditions. The clustering of hydrogen in cation vacancies can be a precursor for the deleterious effects of hydrogen on the mechanical properties and stability of metal oxides, in analogy with hydrogen embrittlement in metals. Finally we observed a red-shift and a blue-shift for the vibrational frequencies of all the hydroxyl groups and all the hydrogen molecules, respectively, in T-ZrO2 when compared to the gas phase frequencies. This is an important characteristic for guiding future experimental efforts to detect and identify hydrogen defects in T-ZrO2. The insights presented in this work advance our predictive understanding of the degradation behavior of T-ZrO2 as a corrosion resistant passive layer, as a gate dielectric and in biomedical applications.
they exist in the form of a hydrogen molecule. In addition, we found that up to three hydrogen species can favorably accumulate in a zirconium vacancy under oxidizing conditions. The clustering of hydrogen in cation vacancies can be a precursor for the deleterious effects of hydrogen on the mechanical properties and stability of metal oxides, in analogy with hydrogen embrittlement in metals. Finally we observed a red-shift and a blue-shift for the vibrational frequencies of all the hydroxyl groups and all the hydrogen molecules, respectively, in T-ZrO2 when compared to the gas phase frequencies. This is an important characteristic for guiding future experimental efforts to detect and identify hydrogen defects in T-ZrO2. The insights presented in this work advance our predictive understanding of the degradation behavior of T-ZrO2 as a corrosion resistant passive layer, as a gate dielectric and in biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...