On the character of the cyclic ionic H-bond in cryogenically cooled deprotonated cysteine†
Abstract
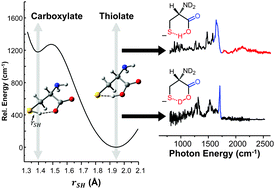
The gas phase structure of deprotonated cysteine (Cys–H+)− has recently gained attention because of its counterintuitive calculated minimum energy structure in which it appears that deprotonation occurs at the –SH moiety rather than at the nominally more acidic carboxylic acid group. Because previous experimental efforts have not yielded to a consensus regarding the structure of the anion, we report the cryogenic ion vibrational predissociation (CIVP) spectra of its cryogenically cooled H/D isotopologues in an effort to clarify the situation. The unexpected isotope dependence of key features in the spectrum and the similarity of the band pattern to that displayed by the intramolecular H-bonded linkage in a deprotonated diacid (HCO2(CH2)10CO2−) indicate that the dominant form of the anion occurs with a strongly shared proton between the thiolate (–S−) and carboxylate (–CO2−) groups. An interesting aspect of this (–S−⋯H+⋯−O2C–) linkage is that, although the global minimum places the shared proton closer to the oxygen atom, the soft potential energy curve calculated for displacement of the bridging proton would likely support sufficient zero-point motion both to blur the distinction between thiolate- and carboxylate-based structures and to account for the unusual isotope effects.

 Please wait while we load your content...
Please wait while we load your content...