Spin–orbit corrected potential energy surface features for the I (2P3/2) + H2O → HI + OH forward and reverse reactions
Abstract
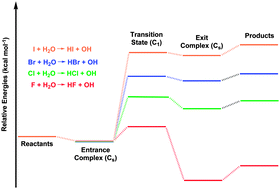
Using the CCSD(T) method with relativistic correlation consistent basis sets up to cc-pVQZ-PP, the entrance complex, transition state, and exit complex for the endothermic reaction I + H2O → HI + OH have been studied. The vibrational frequencies and the zero-point vibrational energies of the five stationary points for the title reaction are reported and compared with the limited available experimental results. Opposite to the valence isoelectronic F + H2O system, but similar to the Cl + H2O and Br + H2O reactions, the I + H2O reaction is endothermic, in this case by 46 kcal mol−1. After the zero-point vibrational energy and spin–orbit coupling corrections, the endothermic reaction energy is predicted to be 48 kcal mol−1, which agrees well with experimental values. For the reverse reaction HI + OH → I + H2O the transition state lies below the reactants, consistent with the experimental negative temperature dependence for the rate constants.

 Please wait while we load your content...
Please wait while we load your content...