Deciphering the infrared spectrum of the protonated water pentamer and the hybrid Eigen–Zundel cation†
Abstract
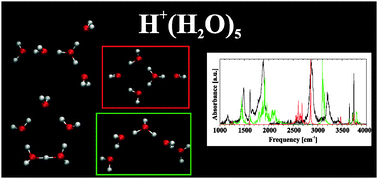
Traditionally, infrared band assignment for the protonated water clusters, such as H+(H2O)5, is based on their lowest energy isomer. Recent experiments extend the observation spectral window to lower frequencies, for which such assignment appears to be inadequate. Because this hydrogen-bonded system is highly anharmonic, harmonic spectral calculations are insufficient for reliable interpretation. Consequently, we have calculated the IR spectrum of several isomers of the protonated water pentamer using an inherently anharmonic methodology, utilizing dipole and velocity autocorrelation functions computed from ab initio molecular dynamic trajectories. While the spectrum of H+(H2O)5 is universally assumed to represent the branched Eigen isomer, we find a better agreement for a mixture of a ring and linear isomers. The first has an Eigen core and contributes at high frequencies, whereas the latter accounts for all prominent low-frequency bands. Interestingly, its core is neither a classical Eigen nor a Zundel cation, but rather has hybrid geometry. Such an isomer may play a role in proton conductance along short proton wires.

 Please wait while we load your content...
Please wait while we load your content...