Relaxation dynamics of photoexcited resorcinol: internal conversion versus H atom tunnelling
Abstract
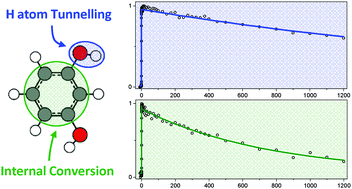
The excited state dynamics of resorcinol (1,3-dihydroxybenzene) following UV excitation at a range of pump wavelengths, 278 ≥ λ ≥ 255 nm, have been investigated using a combination of time-resolved velocity map ion imaging and ultrafast time-resolved ion yield measurements coupled with complementary ab initio calculations. After excitation to the 11ππ* state we extract a timescale, τ1, for excited state relaxation that decreases as a function of excitation energy from 2.70 ns to ∼120 ps. This is assigned to competing relaxation mechanisms. Tunnelling beneath the 11ππ*/1πσ* conical intersection, followed by coupling onto the dissociative 1πσ* state, yields H atoms born with high kinetic energy (∼5000 cm−1). This mechanism is in competition with an internal conversion process that is able to transfer population from the photoexcited 11ππ* state back to a vibrationally excited ground state, S0*. When exciting between 264–260 nm a second decay component, τ2, is observed and we put forth several possible explanations as to the origins of τ2, including conformer specific dynamics. Excitation with 237 nm light (above the 11ππ*/1πσ* conical intersection) yields high kinetic energy H atoms (∼11 000 cm−1) produced in ∼260 fs, in line with a mechanism involving ultrafast coupling between the 11ππ* (or 21ππ*) and 1πσ* state followed by dissociation. The results presented highlight the profound effect the presence of additional functional groups, and more specifically the precise location of the functional groups, can have on the excited state dynamics of model heteroaromatic systems following UV excitation.
- This article is part of the themed collection: Imaging molecular dynamics

 Please wait while we load your content...
Please wait while we load your content...