Joint experimental and computational 17O solid state NMR study of Brownmillerite Ba2In2O5†
Abstract
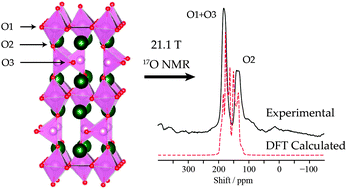
Structural characterization of Brownmillerite Ba2In2O5 was achieved by an approach combining experimental solid-state NMR spectroscopy, density functional theory (DFT) energetics, and GIPAW NMR calculations. While in the previous study of Ba2In2O5 by Adler et al. (S. B. Adler, J. A. Reimer, J. Baltisberger and U. Werner, J. Am. Chem. Soc., 1994, 116, 675–681), three oxygen resonances were observed in the 17O NMR spectra and assigned to the three crystallographically unique O sites, the present high resolution 17O NMR measurements under magic angle spinning (MAS) find only two resonances. The resonances have been assigned using first principles 17O GIPAW NMR calculations to the combination of the O ions connecting the InO4 tetrahedra and the O ions in equatorial sites in octahedral InO6 coordination, and to the axial O ions linking the four- and six-fold coordinated In3+ ions. Possible structural disorder was investigated in two ways: firstly, by inclusion of the high-energy structure also previously studied by Mohn et al. (C. E. Mohn, N. L. Allan, C. L. Freeman, P. Ravindran and S. Stølen, J. Solid State Chem., 2005, 178, 346–355), where the structural O vacancies are stacked rather than staggered as in Brownmillerite and, secondly, by exploring structures derived from the ground-state structure but with randomly perturbed atomic positions. There is no noticeable NMR evidence for any substantial occupancy of the high-energy structure at room temperature.

 Please wait while we load your content...
Please wait while we load your content...