Hydrogen-bonded complexes upon spatial confinement: structural and energetic aspects†
Abstract
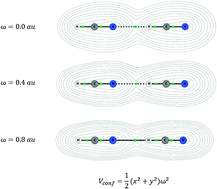
In the present study we consider structural and energetic aspects of spatial confinement of the H-bonded systems. The model dimeric systems: HF⋯HF, HCN⋯HCN and HCN⋯HCCH have been chosen for a case study. Two-dimensional harmonic oscillator potential, mimicking a cylindrical confinement, was applied in order to render the impact of orbital compression on the analyzed molecular complexes. The calculations have been performed employing the MP2 method as well as the Kohn–Sham formulation of density functional theory. In the latter case, two exchange–correlation potentials have been used, namely B3LYP and M06-2X. The geometries of studied complexes have been optimized (without any constraints) in the presence of the applied model confining potential. A thorough analysis of topological parameters characterizing hydrogen bonds upon orbital compression has been performed within the Quantum Theory of Atoms in Molecules (QTAIM). Furthermore, an energetic analysis performed for the confined H-bonded complexes has shown a different trend in the interaction energy changes. Additionally, a variational–perturbational decomposition scheme was applied to study the interaction energy components in the presence of spatial confinement.

 Please wait while we load your content...
Please wait while we load your content...