Role of oxygen vacancies in the surface evolution of H at CeO2(111): a charge modification effect†
Abstract
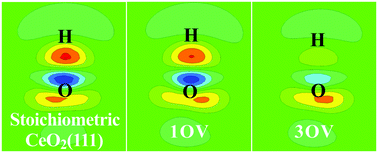
Diffusion processes and reactions of H at stoichiometric and reduced CeO2(111) surfaces have been studied by using density functional theory calculations corrected by on-site Coulomb interactions (DFT + U). Oxygen vacancies on the surface are determined to be able to significantly affect the behavior of H by modifying the charge of surface lattice O through the occurrence of Ce3+. It has been found that, at the reduced CeO2(111) surface, the adsorption strength of H as well as the H coupling barrier can be dramatically reduced compared to those at the stoichiometric surface, while H2O formation barrier is not significantly affected. Moreover, the diffusion of H at the reduced surface or into the bulk can occur more readily than that at stoichiometric CeO2(111).

 Please wait while we load your content...
Please wait while we load your content...