An efficient method to enhance the stability of sulphide semiconductor photocatalysts: a case study of N-doped ZnS†
Abstract
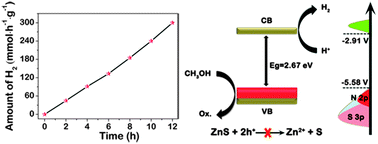
Reducing the oxidative capacity of holes (h+) in the valence band (VB) of ZnS is one of the most effective ways to prevent the photocatalyst from photocorrosion. In this work, ZnS doped only with nitrogen was prepared for the first time by nitriding ZnS powder in an NH3 atmosphere. We demonstrate theoretically and experimentally that the valence band maximum (VBM) rises obviously by N-doping in ZnS, suggesting the reduction of the oxidative capacity of holes (h+) in the valence band. The theoretically predicted band structures were further verified by valence band X-ray photoelectron spectroscopy (VB XPS) and Mott–Schottky measurements. The as-prepared N-doped ZnS exhibited an outstanding stable capability for photocatalytic hydrogen evolution from water under simulated sunlight irradiation for 12 h. However, pristine ZnS showed no capability and was seriously photocorroded under the same conditions.


 Please wait while we load your content...
Please wait while we load your content...