Substrate-controlled band positions in CH3NH3PbI3 perovskite films
Abstract
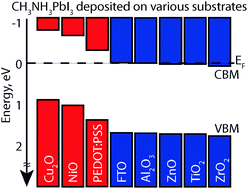
Using X-ray and ultraviolet photoelectron spectroscopy, the surface band positions of solution-processed CH3NH3PbI3 perovskite thin films deposited on an insulating substrate (Al2O3), various n-type (TiO2, ZrO2, ZnO, and F:SnO2 (FTO)) substrates, and various p-type (PEDOT:PSS, NiO, and Cu2O) substrates are studied. Many-body GW calculations of the valence band density of states, with spin–orbit interactions included, show a clear correspondence with our experimental spectra and are used to confirm our assignment of the valence band maximum. These surface-sensitive photoelectron spectroscopy measurements result in shifting of the CH3NH3PbI3 valence band position relative to the Fermi energy as a function of substrate type, where the valence band to Fermi energy difference reflects the substrate type (insulating-, n-, or p-type). Specifically, the insulating- and n-type substrates increase the CH3NH3PbI3 valence band to Fermi energy difference to the extent of pinning the conduction band to the Fermi level; whereas, the p-type substrates decrease the valence band to Fermi energy difference. This observation implies that the substrate's properties enable control over the band alignment of CH3NH3PbI3 perovskite thin-film devices, potentially allowing for new device architectures as well as more efficient devices.

 Please wait while we load your content...
Please wait while we load your content...