Phenothiazine–azaBODIPY–fullerene supramolecules: syntheses, structural characterization, and photochemical studies†
Abstract
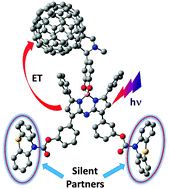
Photoactive supramolecules composed of two entities of carbamoyl phenothiazines (PTZ) positioned at different locations of the BF2-chelated azadipyrromethene (azaBODIPY) periphery and fulleropyrrolidine (C60) have been newly designed and synthesized to probe excited state events. The X-ray structure of one of the precursor compounds, (PTZ)2–azaBODIPY, revealed spatially well separated PTZ entities without causing any steric hindrance. The supramolecules were fully characterized by spectral, computational, electrochemical and photochemical techniques. The geometry and electronic structures were arrived at by B3LYP/6-31G(dp) calculations (for H, B, N, and O) and B3LYP/6-31G(df) calculations (for S) in benzonitrile utilizing the Conductor Polarizable Continuum Model (CPCM). The different redox states were established from the differential pulse voltammetry (DPV) studies and the data were used to estimate free-energy change associated with the charge separation process. Interestingly, although phenothiazine is known to be a very good electron donor in photosynthetic model compounds and solar energy harvesting dyes, in the present system, the difficulty in oxidizing carbamoyl phenothiazine entities' contribution to the photochemistry originating from the singlet excited azaBODIPY was minimal. Consequently, femtosecond laser flash photolysis studies provided evidence for the occurrence of photoinduced electron transfer from 1azaBODIPY* to C60 without participation of the PTZ entities. The energy level diagram constructed using spectral and electrochemical data provided a rational explanation for this observation. By monitoring the rise and decay of the fullerene radical ion peak, the measured rate of charge separation, kCS, and charge recombination, kCR, for supramolecule 1 were found to be 3.08 × 1010 s−1 and 7.96 × 109 s−1, respectively, while these values for supramolecule 2 were found to be 4.68 × 1011 s−1 and 1.13 × 1010 s−1, respectively, revealing the occurrence of ultrafast electron transfer events. The photochemically generated (PTZ)2–azaBODIPY˙+–C60˙− radical ion pair followed a charge recombination path by populating 3azaBODIPY* prior to returning to the ground state as confirmed by nanosecond transient absorption measurements.

 Please wait while we load your content...
Please wait while we load your content...