Absorption spectra of alkali-C60 nanoclusters†
Abstract
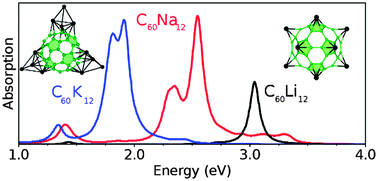
We investigate the absorption spectra of alkali-doped C60 nanoclusters, namely C60Nan, C60Kn, and C60Lin, with n = 1, 2, 6, 12, in the framework of the time-dependent density-functional theory (TDDFT). We study the dependence of the absorption spectra on the nature of the alkali. We show that in few cases the absorption spectra depend on the arrangement of the alkali atoms over the fullerene, though sometimes the absorption spectra do not allow us to distinguish between different configurations. When only one or two alkali atoms are adsorbed on the fullerene, the optical response of alkali-doped C60 is similar to that of the anion C60− with a strong response in the UV domain. In contrast, for higher concentration of alkali, a strong optical response is predicted in the visible range, particularly when metal–metal bonds are formed. The weak optical response of the Ih-symmetry C60Li12 is proposed to be used as a signature of its structure.

 Please wait while we load your content...
Please wait while we load your content...