Electrochemical reduction of N,N′-thiobisphthalimide and N,N′-dithiobisphthalimide: ejection of diatomic sulfur through an autocatalytic mechanism†
Abstract
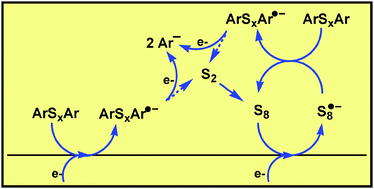
The electrochemical reduction of N,N′-dithiobisphthalimide and N,N′-thiobisphthalimide is investigated using electrochemical techniques and theoretical calculations. The results are rationalized using adequate electron transfer theories. The reduction leads to the ejection of diatomic sulfur and involves an interesting autocatalytic mechanism. This mechanism is dependent on the concentration of the initial compound and the cyclic voltammetric scan rate. The starting material is reduced both at the electrode and through homogeneous electron transfer from the produced sulfur. The initial electron transfer follows a stepwise mechanism involving the formation of the corresponding radical anion. This is supported by both the electrochemical data and the theoretical calculation results. The radical anion of the N,N′-dithiobisphthalimide dissociates through cleavage of the N–S chemical bond and not the S–S chemical bond. Application of the extension of the dissociative electron transfer theory to the dissociation of radical anions shows that the N–S chemical bond dissociates despite being stronger than the S–S chemical bond. This is due to the large difference in the oxidation potentials of the two potential anions (the phthalimidyl anion and the phthalimidyl thiyl anion). The electrochemical reduction of N,N′-thiobisphthalimide involves the intermediate formation of N,N′-dithiobisphthalimide and hence the autocatalytic process is less efficient.

 Please wait while we load your content...
Please wait while we load your content...