Structural properties of iron-phosphate glasses: spectroscopic studies and ab initio simulations
Abstract
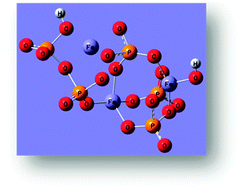
Vitrification is the most effective method for the immobilization of hazardous waste by incorporating toxic elements into a glass structure. Iron phosphate glasses are presently being considered as matrices for the storage of radioactive waste, even of those which cannot be vitrified using conventional borosilicate waste glass. In this study, a structural model of 60P2O5–40Fe2O3 glass is proposed. The model is based on the crystal structure of FePO4 which is composed of [FeO4][PO4] tetrahedral rings. The rings are optimized using the DFT method and the obtained theoretical FTIR and Raman spectra are being compared with their experimental counterparts. Moreover, the proposed model is in very good agreement with X-ray absorption fine structure spectroscopy (XANES/EXAFS) and Mössbauer spectroscopy measurements. According to the calculations the Fe3+ is in tetrahedral and five-fold coordination. The maximal predicted load of waste constituents into the glass without rebuilding of the structure is 30 mol%. Below this content, waste constituents balance the charge of [FeO4]− tetrahedra which leads to their strong bonding to the glass resulting in an increase of the chemical durability, transformation and melting temperatures and density.


 Please wait while we load your content...
Please wait while we load your content...