Formaldehyde chemistry in cometary ices: the case of HOCH2OH formation
Abstract
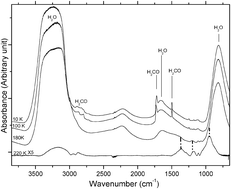
Laboratory experiments devoted to simulate the chemistry occurring in interstellar and cometary ice analogues are of paramount importance to understand the formation of complex organic molecules that are detected throughout the universe. These laboratory simulations provide relevant hints on the fundamental physical and chemical steps associated with the increase of the molecular complexity in space and, moreover, give benchmark results for dedicated space missions. In the present work, we study the thermally promoted reactivity of H2O-dominated and D2O-dominated cometary ice analogues that contain various amounts of H2CO and NH3 by means of Fourier-transform infrared spectroscopy (FTIR), mass spectrometry and DFT calculations. Experimental measurements show that methyleneglycol (HOCH2OH) and D2-methyleneglycol (DOCH2OD, the corresponding isotopologue) are formed from the H2O- and D2O-dominated ices, respectively, only if ammonia is present. We also reported for the first time the mass spectrum of methyleneglycol and D2-methyleneglycol. B3LYP calculations have also been used to characterize the potential energy surface of the mechanistic steps associated with the formation of HOCH2OH as well as to simulate the IR spectrum of this compound. The fruitful interplay between theory and experiment has allowed us to elucidate the exact role of ammonia during the warming, which essentially stands for the formation and stabilization of the NH4+/OH− ion pair, thus enabling the OH− species to react with formaldehyde. The present results reproduce the heating of circumstellar ices in star formation regions and can be applied to the late thermal evolution of comets. In addition, the mass spectrum of methyleneglycol represents a benchmark for the analysis of the data coming from the ROSINA on-board instrument of the Rosetta mission.

 Please wait while we load your content...
Please wait while we load your content...