An electrochemical in situ study of freezing and thawing of ionic liquids in carbon nanopores†
Abstract
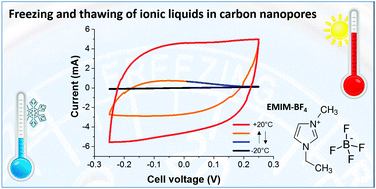
Room temperature ionic liquids (RTILs) are an emerging class of electrolytes enabling high cell voltages and, in return, high energy density of advanced supercapacitors. Yet, the low temperature behavior, including freezing and thawing, is little understood when ions are confined in the narrow space of nanopores. This study shows that RTILs may show a tremendously different thermal behavior when comparing bulk with nanoconfined properties as a result of the increased surface energy of carbon pore walls. In particular, a continuous increase in viscosity is accompanied by slowed-down charge–discharge kinetics as seen with in situ electrochemical characterization. Freezing reversibly collapses the energy storage ability and thawing fully restores the initial energy density of the material. For the first time, a different thermal behavior in positively and negatively polarized electrodes is demonstrated. This leads to different freezing and melting points in the two electrodes. Compared to bulk, RTILs in the confinement of electrically charged nanopores show a high affinity for supercooling; that is, the electrode may freeze during heating.

 Please wait while we load your content...
Please wait while we load your content...