The effects of alloying and segregation for the reactivity and diffusion of oxygen on Cu3Au(111)
Abstract
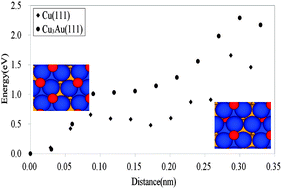
We report results of the segregation induced by the adsorption of O2 and the barrier of the formation of Cu2O in Cu3Au(111) with an experimental and theoretical approach. Oxidation by a hyperthermal O2 molecular beam (HOMB) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. From the incident-energy dependence of the measured O-uptake curve, dissociative adsorption of O2 is less effective on Cu3Au(111) than on Cu(111). The dissociative adsorption is accompanied by the Cu segregation on Cu3Au(111) as well as on Cu3Au(100) and Cu3Au(110). The obvious growth of Cu2O for a 2.3 eV HOMB cannot be observed and it suggests that the Au-rich protective layers prevent the diffusion of O atoms into the bulk. The theoretical approach based on density functional theory indicates that O adsorption shows the same behavior on Cu3Au(111) and on Cu(111). For the diffusion case, the barrier to diffuse into the subsurface in segregated Cu3Au(111) is higher than in Cu(111). This indicates that the segregated Au-rich layer in Cu3Au(111) works as a protective layer.

 Please wait while we load your content...
Please wait while we load your content...