Identification of synergistic Cu/V redox pair in VCu:AlPO-5; a comparison with VCu:ZSM-5†
Abstract
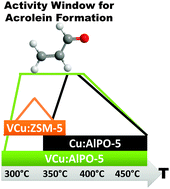
Vanadium(V) and copper(II) were co-deposited into AlPO-5 and H–ZSM-5 three-dimensional microporous carriers to yield VCu:AlPO-5 and VCu:ZSM-5. The materials, along with copper analogues were tested for the selective oxidation of propene, and the catalysts perform in the following order: VCu:AlPO-5 > Cu:AlPO-5 > VCu:ZSM-5 > Cu:ZSM-5. Acrolein was selectively formed over VCu:AlPO-5 and Cu:AlPO-5 over a very wide range from 300 to 450 °C, whereas VCu:ZSM-5 displays a limited temperature window for acrolein formation (300–350 °C). Hence, the choice of carrier and presence of vanadium as a co-cation greatly affects the acrolein selectivity and activity window. The vanadium and copper reduction events were monitored by in situ X-ray Absorption Spectroscopy (XAS) during C3H6-TPR (1.11%) to 450 °C. The Cu(II)/(I) redox pair initiates reduction of V(V) → V(IV) in VCu:AlPO-5 and VCu:ZSM-5 at 375 °C. Metallic copper is the major valence fraction above 400 °C in both samples while vanadium is present as V(IV)/V(III) species. In the monometallic copper analogues Cu(I) is the major valence fraction above 350 °C, hence synergistic effects between the Cu/V pair causes hyper-reduction of copper. EXAFS shows that copper and vanadium are in close proximity in VCu:AlPO-5 only, being linked by bridging oxygens (Cu–O–V) believed to interact with propene. By contrast, propene adsorbs on Brønsted sites in VCu:ZSM-5 inhibiting acrolein formation at elevated temperatures, as confirmed by DRIFTS. We believe the reactive Cu/V pair in neutral AlPO-5 generates extralattice oxygens favouring acrolein formation over a wide temperature range.

 Please wait while we load your content...
Please wait while we load your content...