A comparison between QM/MM and QM/QM based fitting of condensed-phase atomic polarizabilities
Abstract
Recently we reported a combined QM/MM approach to estimate condensed-phase values of atomic polarizabilities for use in (bio)molecular simulation. The setup relies on a MM treatment of the solvent when determining atomic polarizabilities to describe the response of a QM described solute to its external electric field. In this work, we study the effect of using alternative descriptions of the solvent molecules when evaluating atomic polarizabilities of a methanol solute. In a first step, we show that solute polarizabilities are not significantly affected upon substantially increasing the MM dipole moments towards values that are typically reported in literature for water solvent molecules. Subsequently, solute polarization is evaluated in the presence of a QM described solvent (using the frozen-density embedding method). In the latter case, lower oxygen polarizabilities were obtained than when using MM point charges to describe the solvent, due to introduction of Pauli-repulsion effects.
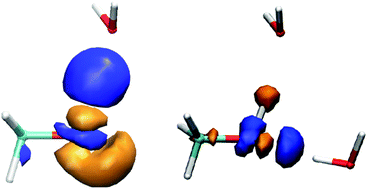

 Please wait while we load your content...
Please wait while we load your content...