A double-QM/MM method for investigating donor–acceptor electron-transfer reactions in solution†
Abstract
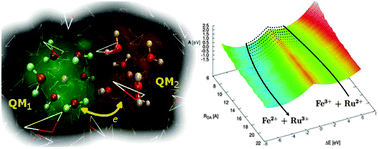
We developed a double-quantum mechanical/molecular mechanical (d-QM/MM) method for investigation of full outer-sphere electron transfer (ET) processes between a donor and an acceptor (DA) in condensed matter. In the d-QM/MM method, which employs the novel concept of multiple QM regions, one can easily specify the number of electrons, spin states and appropriate exchange–correlation treatment in each QM region, which is especially important in the cases of ET involving transition metal sites. We investigated Fe2+/3+ self-exchange and Fe3+ + Ru2+ → Fe2+ + Ru3+ in aqueous solution as model reactions, and demonstrated that the d-QM/MM method gives reasonable accuracy for the redox potential, reorganization free energy and electronic coupling. In particular, the DA distance dependencies of those quantities are clearly shown at the density functional theory hybrid functional level. The present d-QM/MM method allows us to explore the intermediate DA distance region, important for long-range ET phenomena observed in electrochemistry (on the solid–liquid interfaces) and biochemistry, which cannot be dealt by the half-reaction scheme with the conventional QM/MM.

 Please wait while we load your content...
Please wait while we load your content...