Insights into the Cr(iii) catalyzed isomerization mechanism of glucose to fructose in the presence of water using ab initio molecular dynamics†
Abstract
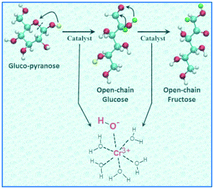
The mechanism of glucose ring opening and isomerization to fructose, catalyzed by the Lewis acid catalyst CrCl3 in the presence of water, is investigated using Car–Parrinello molecular dynamics with metadynamics. Minimum energy pathways for the reactions are revealed and the corresponding free energy barriers are computed. Addition of glucose replaces two water molecules in the active [Cr(H2O)5OH]+2 complex, with two hydroxyl groups of glucose taking their place. Ring opening and isomerization reactions can only proceed if the first step involving the deprotonation of glucose is accompanied by the protonation of the OH− group in the partially hydrolyzed metal center ([Cr(C6H12O6)(H2O)3OH]+2 → [Cr(C6H11O6)(H2O)4]+2). This provides further evidence that the partially hydrolyzed [Cr(H2O)5OH]+2 is the active species catalyzing ring opening and isomerization reactions and that unhydrolyzed Cr+3 may not be able to catalyze the reactions. After the ring opening, the isomerization reaction proceeds via deprotonation, followed by hydride shift and the back donation of the proton from the metal complex to the sugar. Water molecules outside the first coordination sphere of the metal complex participate in the reaction for mediating the proton transfer. The hydride shift in the isomerization is the overall rate limiting step with a free energy barrier of 104 kJ mol−1. The simulation computed barrier is in agreement with experiments.

 Please wait while we load your content...
Please wait while we load your content...