Hydrophobic dipeptide crystals: a promising Ag-free class of ultramicroporous materials showing argon/oxygen adsorption selectivity†
Abstract
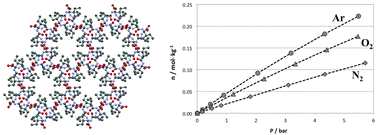
The adsorption isotherms of nitrogen, oxygen and argon in four VA-class hydrophobic dipeptides are presented. Isotherms were determined at 5, 20 and 35 °C, for a pressure range of 0–6 bar. Under these conditions, adsorption is still in the Henry region. For all materials and temperatures, the sequence of preferential adsorption is Ar > O2 > N2, a highly abnormal result. At 5 °C, the dipeptide with the smallest pores, VI, has Ar/O2 adsorption equilibrium selectivities up to 1.30, the highest ever measured in Ag-free adsorbents. Gas uptakes, at 1 bar and 20 °C, are ∼0.05 mol kg−1, very low relative values that are partially explained by the low porosity of the solids (<10%). The significance of these results for the development of new materials for the process of O2 generation by pressure swing adsorption (PSA) is discussed. The results indicate some of the structural and chemical properties that prospective Ag-free adsorbents should have in order to have Ar/O2 selectivity, hydrophobic pores, less than 0.5 nm-wide, and porosity of, at least, 20%.

 Please wait while we load your content...
Please wait while we load your content...